Janssen Vaccine Booster Approval
A single booster dose of the Janssen COVID-19 Vaccine 05 mL may be administered as a heterologous booster dose following completion of primary vaccination with another authorized or approved. On Thursday the US.
/cloudfront-us-east-2.images.arcpublishing.com/reuters/EGJVBEQTQZPJTJWV6IMJ67F23Q.jpg)
U S Fda Advisers To Vote On J J Vaccine Booster Reuters
An influential Centers for Disease Control and Prevention advisory committee earlier Thursday unanimously endorsed boosters of Modernas and Johnson Johnsons Covid-19 vaccines.

Janssen vaccine booster approval
. The Centers for Disease Control and Prevention CDC has approved vaccine boosters for all adults who received a Johnson Johnson COVID-19 vaccine and certain high-risk adults who received the Moderna vaccine. October 15 2021 545 PM EDT. Fink recapping the FDAs decision yesterday to authorize heterologous COVID-19 booster doses of the Pfizer-BioNTech Moderna and Janssen vaccines. For the unregistered vaccines that are granted recognition effective vaccination would be considered to extend from 14 days after the last dose of the schedule which is currently two doses except for Janssen but may be a booster doses six to twelve months after the last dose of the schedule.A single booster dose of the Janssen COVID-19 Vaccine may be administered to eligible individuals who have completed primary vaccination with a different authorized or approved COVID-19 vaccine. The JJJanssen COVID-19 vaccine has lower vaccine effectiveness over time compared to mRNA COVID-19 vaccines Pfizer-BioNTech and Moderna. On October 15 2021 the Janssen team requested the Authorization of a booster dose for people 18 and older six months after the initial one-dose immunization with an option to offer vaccinations after two months depending on local conditions and the needs of specific groups of people. Unlike both the Pfizer-BioNTech and Moderna boosters which are recommended at least six.
T he Food and Drug Administrations FDA expert vaccine panel on Friday Oct. Food and Drug Administration FDA approved the use of the Moderna and Janssen Johnson Johnson COVID-19 vaccines as a booster dose after their primary versions are completed. Approved vaccines and list of countries and territories with approved COVID-19 proof of vaccination for travel to England. JJ To File This Week For Booster Shot Approval Johnson Johnson plans to apply early this week to the Food and Drug Administration for authorization to administer a second dose of.
JJ seeks US. The FDA is now assessing whether Americans who originally received the Janssen or Moderna vaccine should get a booster shot. JJ-Janssens vaccine is the only one that requires a single dose. The FDA amended the emergency use authorizations EUAs for.
Boosting Janssens vaccine two months after the first shot increased antibody response fourfold the company said and was associated in real life. On October 20 2021 the FDA authorized a single booster dose of the Janssen Johnson and Johnson COVID-19 Vaccine administered at least 2. Also part of the submission is Phase 12a data showing that when a booster of the Johnson Johnson COVID-19 vaccine was given six months after the single shot antibody levels increased nine-fold one week after the booster and continued to climb to 12-fold higher four weeks after the booster. People ages 18 years and older who received a JJJanssen COVID-19 vaccine at least 2 months ago should get a booster shot.
On Wednesday the FDA announced that limited data submitted by Johnson Johnson. The Janssen booster second vaccine was approved for persons 18 years and older for 2 or more months after receiving the initial dose. COVID-19 Vaccine Janssen is made up of another virus of the adenovirus family that has been modified to contain the gene for making a protein found on SARS-CoV-2. With those data in hand CDC will keep the public informed with a timely plan for JJJanssen booster shots.
The vaccine when given as a booster or primary dose was generally well-tolerated. Booster recipients do not need to get the same vaccine as used in their primary series a practice referred to as mix-and-match. COVID-19 Vaccine Janssen is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 18 years and older. This is based on generalising the data from duration of immunity studies reviewed in the.
COVID-19 is caused by SARS-CoV-2 virus. A single booster dose of the Janssen COVID-19 Vaccine may be administered at least two months after primary vaccination with the Janssen COVID-19 Vaccine. The FDA authorized Covid vaccine booster shots made by JJ and Moderna another critical step in distributing extra doses to tens of millions of people. A box of vials of the new vaccine by Janssen Pharmaceuticals a company owned by Johnson Johnson.
The meeting began with Dr. More data on the effectiveness and safety of JJJanssen booster shots are expected soon. Please check with your health. At this time people who got the JJJanssen vaccine are not eligible for a booster shot.
At the Union Plaza Apartments Governor. 15 recommended a booster dose of. The authorization for emergency use of a single booster dose of the Janssen COVID-19 Vaccine is based on the FDAs evaluation of immune response data in 39 participants from a clinical trial. Approval for COVID vaccine booster doses.
The Janssen COVID-19 Vaccine has not been approved or licensed by the FDA but has been authorized for emergency use by the FDA under an EUA for active immunization to prevent Coronavirus Disease 2019 COVID-19 in individuals 18 years of age and older. The American health service FDA approved a third shot of the Pfizer vaccine in September for vulnerable individuals such as the elderly or those with weakened immune systems. A single Janssen COVID -19 Vaccine booster dose 05 mL may be administered at least 2 months after primary vaccination with the Janssen COVID -19 Vaccine to individuals 18 years of age and older. Health Secretary Francisco Duque III has approved the recommendation of an independent panel to give COVID-19 booster shots and additional vaccine.

J J Seeks Fda S Approval For Covid 19 Vaccine Booster Shot In Adults Business Standard News

Fda Oks Mixing Covid Vaccines Backs Moderna J J Boosters
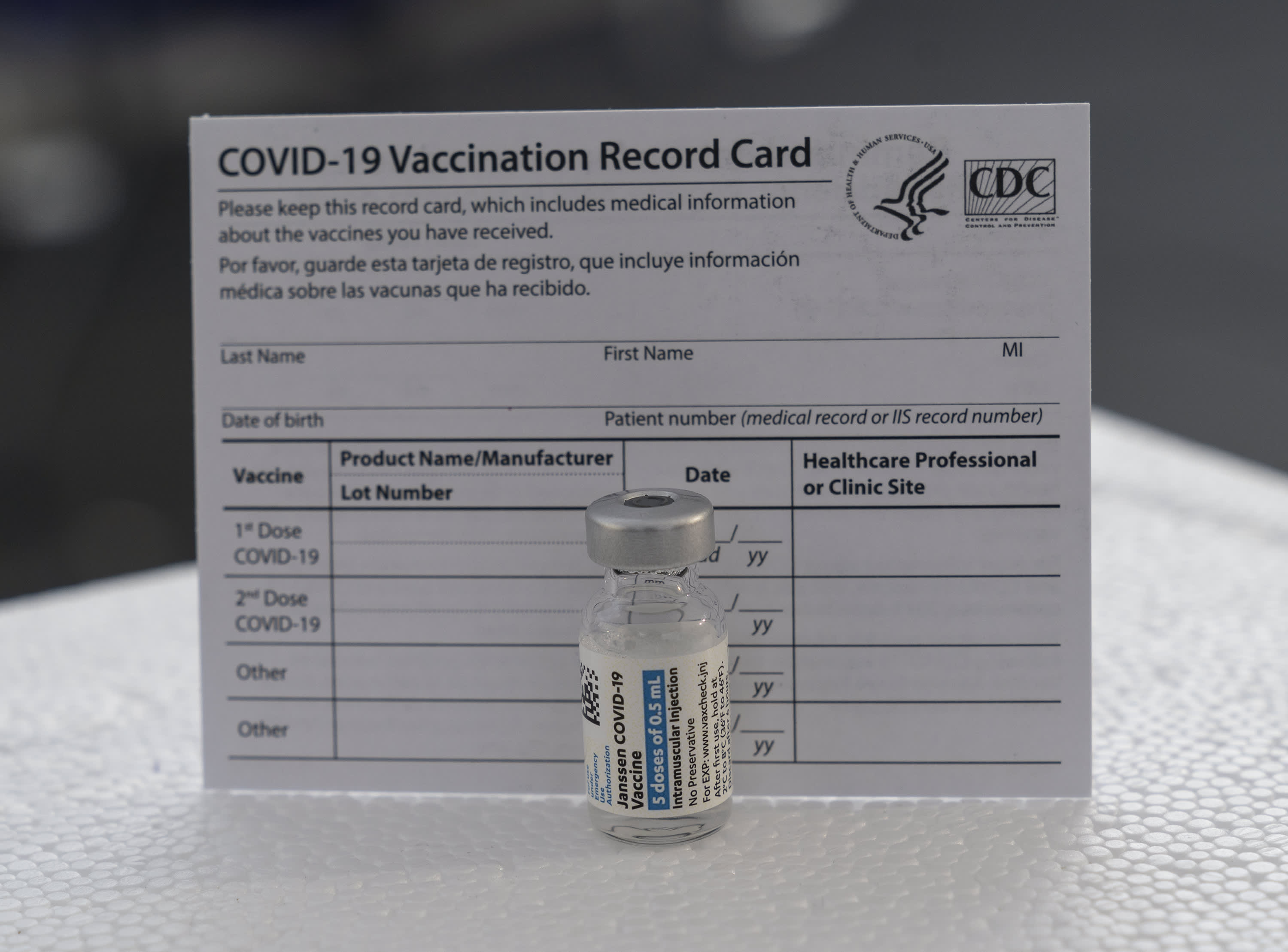
What People Who Got J J Covid Vaccine Need To Know About Boosters

Fda Advisory Panel Votes 19 0 To Endorse Booster Dose Of J J Vaccine Stat
Johnson Johnson Asks Fda For Approval Of Covid Vaccine Booster Shots Syracuse Com
Fda Panel Endorses J J Covid 19 Booster Shot

J J Seeks Usfda Approval For Covid 19 Vaccine Booster Doses
So You Got The J J Vaccine Here S What You Should Know About The Delta Variant Boosters And More Aamc
Post a Comment for "Janssen Vaccine Booster Approval"